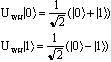Quantum
Computing
Alexander
Laurich
Basic Intro to Quantum
Mechanics
- Does not obey classical laws
- Governed by Probability
- Nothing real until an observation has
been made
Particle Nature
of Light
- Newton and many other scientists view
light as being a tiny particle. They drew this conclusion from the basic
behaviors of light. The primary characteristics of light are that light
bounces off surfaces in the same way a ball would, and light travels in a
straight line as particles do. The photoelectric effect also gave evidence to
the fact that light was a particle.
The Photo Electric Effect
- Phillip Lenard discovered that
electrons are emitted when light is shined upon a metallic surface. This was
called the photoelectric effect. He hypothesized that if brighter light were
used the electrons would fly off with a higher velocity. But he found that the
electrons did not fly off any faster, just more electrons flew off. This holds
true only if the frequency of the light remains constant.
- Einstein explained this fact using
Plank’s equation of E=hv. He stated that light was not a wave, it was indeed a
particle. It comes in packets of energy. Every time such a light quanta hits
the metallic surface, it ejects an electron. Increasing the brightness merely
increases the number of light quanta, not the energy of each photon.
Wave Nature of Light
- Physicist Chistiaan Huygens came up
with the idea that light was a wave and not a particle. But at the time the
main observed effects of light could only be explained it light were a
particle
Double Slit
Experiment with Light
- This experiment was devised by
Physicist Thomas Young to test whether or not light acts as a wave, similar to
water. If two circular, non-concentric ripples of water meet, one will observe
the process of interference. Where the crests or the troughs of the ripples
meet, the magnitude will add, but where the crest of one meets the trough of
another, the water will appear flat.
- Young tested this theory in regards to
light by shooting light through a double slit and observing the pattern it
projected on a surface. He found that light produces an interference pattern
not simply a projection of the two slits. This provided evidence that light
was surely a wave.
Quantum Nature of Particles
- Quantum theory largely comes from the
concept that energy in terms of atoms is not emitted in any amount, but is
limited to an exact piece of energy (quanta). This was the beginning of a
theory to unify the wave nature and particle nature of light
Blackbody Radiation
- Blackbody radiation is the phenomenon
of hot objects glowing with different distinct colors. In the early 1900s
scientists tried to explain this by saying that as an object gets hotter, the
electrons are excited and release a stream of photons. Classical physics said
that as the temperature increase towards infinity, the frequency of the light
emitted would also go to infinity. Instead scientists found that the light cut
off after a certain point.
- Plank to the challenge of explaining
this deviation. His formula fit the radiation curve nicely. He found that as
the frequency of the light increases, more and more energy was required to
eject photons. At incredibly high frequencies large amounts of energy is
required to release more quanta so the amount released drops
drastically.
Wave-Particle
- Many experiments showed that light
behave as both a particle and a wave. Thus physicists decided that a
wave-particle duality exists as part of nature. Many seemed worried about this
possibility, but needlessly. Nature has no problem with light being both a
particle and a wave. It is only confusing when we view light as a particle and
a wave
- This led to deBroglie’s conclusion that
all matter posses both a wave state and a matter state. This follows the
principle of complementarity which says that one can not measure both the wave
characteristic and the particle characteristic of matter. If one performs an
experiment to test wave nature, one will find a wave. And if one performs an
experiment for particle nature one will find a particle
More on the Double Slit
Experiment (with Electrons)
- Based off of the wave-particle duality
conclusion, think about shooting bullets through the double slit. If one tests
for the probability that a bullet will go through the right slit and likewise
for the other slit. The probability of the bullet hitting the detector is the
sum of the pervious probabilities.
- On the other hand, if this experiment
is done using electrons, the probability of the electron hitting the detector
is not the sum of the probabilities. This effect comes from interference. We
are now dealing with the probability function of the electron. To obtain the
probability of the electron hitting the detector, one must not square the wave
function
- There is one special change that can be
made to the experiment to completely change the results. If a measurement is
taken at the point the particles enter the double slit, the location of the
electrons is exactly known and the wave function collapses. One will no longer
see a diffraction pattern on the detector. This effect is due to uncertainty
and the collapse of superposition.
Uncertainty Principle
- This principle states that we can never
know a particle’s location and speed with infinite accuracy, If we know a
particle’s speed with infinite precision, we have no clue where it is. The
same holds true for knowing a particle’s position. This principle can be
explained in physical terms. In order to see something, a photon must impart
some energy to it. We now know where the particle is, but the collision with
the photon affected the particle’s velocity.
- Uncertainty can be thought of as the
protective principle of quantum mechanics. If one could measure position and
momentum at the same time, quantum mechanics would collapse. Many have tried
clever ways to defeat this principle, but no one has succeeded.
Superposition
- The concept of uncertainty leads to the
idea of superposition. This says that an unknown particle obeys a certain
probability function. In other words a particle only has a probability of
being in a certain state. This comes from an extension of the idea of the
double slit experiment with electrons. The following equation describes the
wave function y. Where the probability of
getting either a 0 or a 1 is determined by a and b. Where a and be must
satisfy the following equation.
\phi = a\ket0 + b\ket1 where abs(a)^2 +
abs(b)^2 = 1
- From this concept on can see that for
the probability of getting either a 0 or a 1 to be equal a = b. Thus:
a = b = 1/sqrt(2)
This describes a common initial
superposition in quantum computing.
Entanglement
- This is one of the more amazing effects
in quantum mechanics. Two particles can become entangled and become one
system. Then if one of the particles is disturbed, the other one is also
affected by this perturbation.
- For example: an atom releases two
photons polarized in exactly different ways. Let’s say this pair is entangled
to the same system. Until one measures one of the photons both of their states
are unknown. But when an observer measures one of the photons, its
polarization is defined. And because the two are entangled, the state of the
other photon will then be defined as well. This change is completely
independent of distance.
Non-locality
- Interaction between two entangled
particles is completely independent of the distance between the particles.
This action is attributed to non-locality. This eerie phenomenon allows an
instantaneous change in a particle even if it is in another galaxy. This
concept has been used to test teleportation, and been done
successfully.
Different
Views of Quantum Mechanics
- Schrodinger’s Cat
- A basic example of quantum mechanics is
that of Schrodinger’s Cat. Schrodinger proposed a thought experiment in which
a cat was place in a room that contains a closed vial of poison and a
radioactive particle. This radioactive atom has a 50% chance of decaying at
any time. When it does decay it will open the vial of poison and the cat will
die
- You have now created a superposition of
a live cat and a dead cat. The cat can no longer be described by classical
methods, but only by a probability function.
- Copenhagen
- The Copenhagen interpretation of
quantum mechanics involves the abstract view of particles. This idea says that
we cannot say what a particle does, even if it does exist while we are not
looking at it. This basically says that a microscopic particle does not exist
until we observe it (interact with it).
- Many Worlds
- The many worlds theory gets rid of the
idea of probability and wave functions and reduces it to alternate worlds. The
cat is both dead and alive, and both possibilities are equally real. When we
make an observation, we are forced to decide between the two different worlds
that then becomes real for us.
- On the surface this may seem like
science fiction, but this is merely the consequence of taking the ideas of
quantum mechanics literally.
Basics of Quantum
Computers
The Qubit
- The basic unit to a computer is the
bit. A digital computer uses Shannon bits. These bits can only represent a 0
or a 1 at a given moment. This only allows one number to be represented by any
number of bits.
- A quantum computer uses qubits. These
bits are described by some state of the system. Often a spin state or a
polarization. Generally these states are denoted by
![]() or
or ![]() . What makes qubits so
special is superposition. This allows a qubit to have a probability of being
either a 0 or a 1. This allows a set of n qubits to represent 2n
different numbers simultaneously.
. What makes qubits so
special is superposition. This allows a qubit to have a probability of being
either a 0 or a 1. This allows a set of n qubits to represent 2n
different numbers simultaneously.
- This leads to the idea of quantum
parallelism. If a qubit is in superposition, it is acting as both a zero and a
one. This allows computations to be performed simultaneously as if the
computer were computing a given function for all possible initial states.
Thought there is some limitation as to how the results can be measured.
Measurement
- One cannot read off a quantum computer
in the way one can read the state of a normal computer. If one reads off a
qubit in superposition, the qubit will collapse into one of the possible
solutions. This renders the calculation point less because it is then no more
efficient than a normal computer. The parallelism of the computer will have
stopped.
Decoherence
- Every interaction with the environment
constitutes a measurement. For this reason, one must isolate the quantum
computer. Otherwise the quantum states will collapse from superposition and
all calculations will be wrong. This problem is commonly referred to as
decoherence. If the accidental photon hits the system, the quantum states will
collapse for what was hit.
Gates
- A quantum computer utilizes gates like
any other computer does. But these gates are not based on transistors and
electrical circuits which direct electron flow; these gates are generally
based on laser manipulation, rf field manipulation, or application of
NMR.
- The implementation of the following
gates is very complex and their description is mostly limited to mathematical
discussion, but following this section two examples of actual quantum
computers are provided.
- One very important requirement of
quantum gates is that they are completely reversible. This is due to the fact
that no energy change can occur during calculation. Classical systems
dissipate heat during computation, but a quantum computer can not if it is to
maintain superposition.
- Walsh-Hadamard Gate
- This is one of the most basic types of
one-qubit gates. This gate puts a state of either
![]() or
or ![]() into a superposition of both
states. This gate can also bring a superposition of sates into a state
of
into a superposition of both
states. This gate can also bring a superposition of sates into a state
of ![]() or
or ![]()
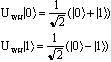
- CNOT
- This is a controlled-not gate. The
basic gate from which basically any other gate can be built. It consists of
one path that allows the first variable to pas through normally, and the
second variable will XOR with the first. Thus we have a gate that will be
reversible and can be expanded to form other gates. The following unitary
transformations show the results of the CNOT gate based on definite
inputs
![]() Examples of Gates
Examples of Gates
![]() Cytosine in D2O
Cytosine in D2O
- This system involves the spin of the
extra protons, those not bonded to nitrogen molecules. The spin of the other
three protons can be ignored because they are involved in rapid exchange to
the solvent the molecule is in. One then has a two-spin system capable of
quantum computation
![]() 13C-labeled Chloroform
13C-labeled Chloroform
- This molecule can be used to create a
two-spin system through the interaction between the proton and the center
carbon atom. One special modification that must be made to the molecule is
changing the carbon. A normal carbon has no spin, so a neutron must be added
to give it spin.
Algorithms
- Grover’s Searching
Algorithm
- Lou Grover devised a revolutionary
algorithm to search unsorted databases. This allows the database to be search
in square root of N calculations instead of N/2 calculations. Which is a huge
increase in speed.
- The mathematics behind the function are
very complicated, but basically the algorithm attempts to amplify the correct
answer. With each iteration of the function, the amplitude of the correct
answer in increased and thus one can find the location of the target
object
- Shor’s Prime Factorization
Algorithm
- This algorithm was recently developed
and poses a great threat to digital encryption and security systems. Many
security systems such as DES and RSA use the difficulty of factoring numbers
to provide security. A normal computer could take months to crack an RSA
key.
- Shor’s algorithm reduces the complexity
of factoring numbers to polynomial time. Now factoring a 400-digit number will
not take in excess of several thousand years, but less that one year. For this
reason, some people are beginning to fear what quantum computers could
accomplish.
- This algorithm is far more complex than
Grover’s algorithm. Simply though, it tries to find the period of a certain
function. Which can be accomplished rather quickly using quantum
parallelism
Examples of
Quantum Computers
- NMR
- This approach to quantum computing
involves the use of a Nuclear Magnetic Resonance device. In a magnetic field,
particles will begin to line up with the field. A high frequency pulse will
rotate certain molecules relative to the magnetic field lines. This allows the
atoms to be put in superposition and then calculation to ensue based on the
conditions of the system. NMR is one way to execute different quantum
gates.
- Ion Entrapment
- Another possibility for quantum
computing is a group of atoms in a linear trap. A laser pulse could then be
directed at one of the atoms to induce it to change spin. This would be
another way to execute quantum gates.
Other applications
- Cryptography by non-clonability
- Teleportation by entanglement and
non-locality